Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 ssbauer spectroscopic study of uranium oxysulfide
ssbauer spectroscopic study of uranium oxysulfideMasaki, Nobuyuki; Nakada, Masami; Akabori, Mitsuo; Arai, Yasuo; Nakamura, Akio; Sato, Nobuaki*
no journal, ,
The U-238 M ssbauer effect of uranium oxysulfide, UOS, has been investigated at various temperatures from 19 K to 80 K. The
ssbauer effect of uranium oxysulfide, UOS, has been investigated at various temperatures from 19 K to 80 K. The  -ray source used for U-238 M
-ray source used for U-238 M ssbauer spectroscopy was Pu-242 dioxide with high isotope purity. By using a pure Ge detector, the 44.9 keV U-238 M
ssbauer spectroscopy was Pu-242 dioxide with high isotope purity. By using a pure Ge detector, the 44.9 keV U-238 M ssbauer
ssbauer  -ray is easily distinguished from the 59.5 keV
-ray is easily distinguished from the 59.5 keV  -ray of the Am-241 isotope, which is the daughter nucleus of the isotopic impurity Pu-241. Quadrupole splitting of the U-238 nucleus in UOS is approximately -40 mm/s in the temperature range without account of magnetic splitting. According to neutron diffraction and magnetic susceptibility measurements, UOS is antiferromagnetic at low temperatures with a Neel temperature of T
-ray of the Am-241 isotope, which is the daughter nucleus of the isotopic impurity Pu-241. Quadrupole splitting of the U-238 nucleus in UOS is approximately -40 mm/s in the temperature range without account of magnetic splitting. According to neutron diffraction and magnetic susceptibility measurements, UOS is antiferromagnetic at low temperatures with a Neel temperature of T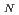 = 55 K. However, M
= 55 K. However, M ssbauer spectra below 55 K could be analyzed by using only quadrupole splitting without magnetic splitting. This result showed that the magnitude of quadrupole splitting was much larger than that of magnetic splitting of U nucleus in UOS.
ssbauer spectra below 55 K could be analyzed by using only quadrupole splitting without magnetic splitting. This result showed that the magnitude of quadrupole splitting was much larger than that of magnetic splitting of U nucleus in UOS.
 F+
F+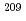 Bi reaction
Bi reactionNishinaka, Ichiro; Kasamatsu, Yoshitaka*; Tanikawa, Masashi*; Goto, Shinichi*; Asai, Masato
no journal, ,
Unexpected steep falloff of fusion cross sections has recently been observed in heavy-ion fusion reactions at sub-barrier energies. In this work, the fusion-fission cross sections of 0.08-650 mb for  F+
F+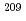 Bi at incident beam energies of 83-135 MeV were determined by a radiochemical method in order to study sub-barrier fusion hindrance. We will discuss the sub-barrier fusion hindrance in the present reaction, comparing theoretical calculations and experimental data.
Bi at incident beam energies of 83-135 MeV were determined by a radiochemical method in order to study sub-barrier fusion hindrance. We will discuss the sub-barrier fusion hindrance in the present reaction, comparing theoretical calculations and experimental data.
Yoshida, Keisuke*; Inoue, Mutsuo*; Minakawa, Masayuki*; Nakano, Yusuke*; Kofuji, Hisaki*; Otosaka, Shigeyoshi; Kiyomoto, Yoko*; Shiomoto, Akihiro*; Hamajima, Yasunori*; Yamamoto, Masayoshi*
no journal, ,
The authors have developed an analytical method for Th-228, Ra-226 and Ra-228 measurements in seawater using an ultra-low background  -ray detector. In this study, the authors measured the three radionuclides in surface seawater obtained from 16 stations in the Japan Sea, East China Sea and Okhotsk Sea. Concentrations of Ra-228 and Th-228 in surface waters in the southern Japan Sea were lower than those in the northern regions. Considering that the Th-228/Ra-228 activity ratios were not different between the northern and southern regions of the Japan Sea, we could conclude that (1) input of terrestrial materials from the East China Sea has not affected the distribution of the radionuclides in the Japan Sea, and (2) the efficiency of scavenging was not different between the regions.
-ray detector. In this study, the authors measured the three radionuclides in surface seawater obtained from 16 stations in the Japan Sea, East China Sea and Okhotsk Sea. Concentrations of Ra-228 and Th-228 in surface waters in the southern Japan Sea were lower than those in the northern regions. Considering that the Th-228/Ra-228 activity ratios were not different between the northern and southern regions of the Japan Sea, we could conclude that (1) input of terrestrial materials from the East China Sea has not affected the distribution of the radionuclides in the Japan Sea, and (2) the efficiency of scavenging was not different between the regions.
Ninomiya, Kazuhiko
no journal, ,
no abstracts in English
 SO
SO /HNO
/HNO mixed solutions; Towards to study on sulfate complexation of
mixed solutions; Towards to study on sulfate complexation of 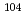 Rf
RfLi, Z.; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Kikuchi, Takahiro; Sato, Nozomi; Nagame, Yuichiro
no journal, ,
Miyamoto, Yutaka; Yasuda, Kenichiro; Magara, Masaaki; Kimura, Takaumi
no journal, ,
For accurate isotopic analysis of trace uranium, chemical separation of tungsten from uranium and plutonium in environmental samples for safeguards with an anion-exchange column was investigated. The mixture of HCl and HF was appropriate for the elimination of tungsten from uranium, and this technique is applicable to the separation of uranium in environmental samples.
Miyamoto, Yutaka; Yasuda, Kenichiro; Magara, Masaaki; Kimura, Takaumi
no journal, ,
Appropriate composition of eluents for sequential separation of trace U, Th, Pb and lanthanides with a single anion-exchange column was examined. The mixtures of acetic acid, hydrochloric acid and nitric acid made complete separation of the elements of interest. The elements in some reference materials were separated with this technique, and performance of this separation was evaluated.
Kokubu, Yoko
no journal, ,
no abstracts in English
Asai, Masato; Tsukada, Kazuaki; Kasamatsu, Yoshitaka*; Sato, Tetsuya; Toyoshima, Atsushi; Nagame, Yuichiro
no journal, ,
We have successfully obtained a large amount of long-lived Fm and Es tracers which can be used in various kinds of heavy-actinide chemistry experiments. Fm and Es isotopes were produced with a Cf target and a  C beam from the JAEA tandem accelerator. Reaction products recoiling out of the target were collected with a gas-jet transport technique. After 3-day irradiation, Fm and Es isotopes were chemically separated from the collected products. By measuring
C beam from the JAEA tandem accelerator. Reaction products recoiling out of the target were collected with a gas-jet transport technique. After 3-day irradiation, Fm and Es isotopes were chemically separated from the collected products. By measuring  - and
- and  -energy spectra, we evaluated an amount of each Fm and Es isotope, and other radioactive impurities. We also performed
-energy spectra, we evaluated an amount of each Fm and Es isotope, and other radioactive impurities. We also performed  -
- coincidence measurements for
coincidence measurements for 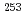 Fm using the same source.
Fm using the same source.
Nagao, Seiya*; Aramaki, Takafumi*; Irino, Tomohisa*; Uchida, Masao*; Shibata, Yasuyuki*; Togawa, Orihiko
no journal, ,
no abstracts in English
Ikeda, Atsushi
no journal, ,
Synchrotron-based analytical methods, such as X-ray absorption spectroscopy (XAS) or X-ray scattering (XS), have recently been recognized as a very powerful tool to investigate the solution/coordination chemistry of chemical substances. These methods have also been applied to the basic- and applied research concerning the actinide species in solution. In this talk, several recent topics related to the actinide speciation study using synchrotrons will be presented, as well as the prospect and future subjects of this research field.
Ikeda, Atsushi
no journal, ,
As compared to typical transition metals, the solution/coordination chemistry of actinides (An) is very unique because of their chemical similarity in the series, as well as their diversity of oxidation states from III to VII. The complex structure of chemical species in solution is the fundamental information to grasp the chemical properties of An in solution. In this study, the structural identity of An species with different oxidation states has been determined in various aqueous solutions by means of synchrotron-based X-ray absorption spectroscopy. The comparison of the obtained results with other reported data provides us several comprehensive trends of the chemical properties of An in aqueous solution.
 I in the Japan Basin and Yamato Basin
I in the Japan Basin and Yamato BasinSuzuki, Takashi; Minakawa, Masayuki*; Togawa, Orihiko
no journal, ,
no abstracts in English
 C measurement by JAEA-AMS-TONO
C measurement by JAEA-AMS-TONOKokubu, Yoko; Nishizawa, Akimitsu*; Owaki, Yoshio*; Nishio, Tomohiro*; Suzuki, Mototaka; Ishimaru, Tsuneari
no journal, ,
no abstracts in English
Kitatsuji, Yoshihiro; Kimura, Takaumi; Kihara, Sorin*
no journal, ,
The behaviors of reduction of NpO
 to Np
to Np or Np
or Np by controlled potential electrolysis were studied, and a unique time course of electrolysis current was observed. It was concluded that NpO
by controlled potential electrolysis were studied, and a unique time course of electrolysis current was observed. It was concluded that NpO
 was reduced by two reduction processes at a platinum electrode: the chemical reaction with Np
was reduced by two reduction processes at a platinum electrode: the chemical reaction with Np and the electrocatalytic reduction by adsorbed hydrogen atom on platinum electrode surface. NpO
and the electrocatalytic reduction by adsorbed hydrogen atom on platinum electrode surface. NpO
 is reduced to Np
is reduced to Np more selectively and with smaller overpotential through electrocatalytic reduction process, though long electrolysis time are required to reduce NpO
more selectively and with smaller overpotential through electrocatalytic reduction process, though long electrolysis time are required to reduce NpO
 completely. To improve the electrolysis efficiency of the preparation of Np
completely. To improve the electrolysis efficiency of the preparation of Np from NpO
from NpO
 , the platinized platinum electrode was applied to bulk electrolysis. The rapid preparation of Np
, the platinized platinum electrode was applied to bulk electrolysis. The rapid preparation of Np comparable to that of reversible redox reaction such as NpO
comparable to that of reversible redox reaction such as NpO
 / NpO
/ NpO
 with small overpotential was achieved successfully.
with small overpotential was achieved successfully.
Arisaka, Makoto; Watanabe, Masayuki; Kimura, Takaumi
no journal, ,
Adsorption behaviors of trivalent actinides (An(III)) and lanthanides (Ln(III)) to activated carbons with and without oxidation by concentrated nitric acid were investigated. Both An(III) and Ln(III) was adsorbed to activated carbons in the range from pH 1 to 4, and the distribution coefficients (
 ) of An(III) and Ln(III) increase with an increase of pH. Activated carbon without oxidation exhibits significant selectivity to An(III) compared with Ln(III). On the other hand, the oxidation to activated carbon deteriorates the selectivity to An(III). The depletion by the oxidation was attributed to the increase of functional groups of oxygen-donor, e.g., carboxyl and/or carbonyl groups because these functional groups are unfavorable for separation between An(III) and Ln(III). The selectivity of activated carbon without oxidation would be attributed to the interaction between
) of An(III) and Ln(III) increase with an increase of pH. Activated carbon without oxidation exhibits significant selectivity to An(III) compared with Ln(III). On the other hand, the oxidation to activated carbon deteriorates the selectivity to An(III). The depletion by the oxidation was attributed to the increase of functional groups of oxygen-donor, e.g., carboxyl and/or carbonyl groups because these functional groups are unfavorable for separation between An(III) and Ln(III). The selectivity of activated carbon without oxidation would be attributed to the interaction between  -electrons of aromatic ring and the metal ions.
-electrons of aromatic ring and the metal ions.
Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Kitatsuji, Yoshihiro; Nagame, Yuichiro; Oe, Kazuhiro*; et al.
no journal, ,
Reduction of element 101, mendelevium (Md), was studied with flow electrolytic chromatography. Mendelevium-255 with a half-life of 27 min was produced in the  Cm(
Cm(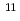 B, 4n) reaction at the JAEA tandem accelerator. Reaction products transported by a KCl/He gas-jet method to the chemistry laboratory were collected on a chemistry apparatus. After removing KCl with HDEHP chromatography, elution behavior of Md in 0.1 M HCl was investigated with a flow electrolytic column apparatus at the applied potentials between 0
B, 4n) reaction at the JAEA tandem accelerator. Reaction products transported by a KCl/He gas-jet method to the chemistry laboratory were collected on a chemistry apparatus. After removing KCl with HDEHP chromatography, elution behavior of Md in 0.1 M HCl was investigated with a flow electrolytic column apparatus at the applied potentials between 0  -0.9 V vs. a Ag/AgCl reference electrode. Elution behavior of Md was the same as that of
-0.9 V vs. a Ag/AgCl reference electrode. Elution behavior of Md was the same as that of 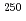 Bk
Bk at 0 V, showing that the stable Md
at 0 V, showing that the stable Md was not reduced to the divalent state. At -0.9 V, elution of Md was quite similar to that of Sr
was not reduced to the divalent state. At -0.9 V, elution of Md was quite similar to that of Sr , demonstrating that Md
, demonstrating that Md was successfully reduced to Md
was successfully reduced to Md .
.
 -ray analysis on J-PARC NOBORU
-ray analysis on J-PARC NOBORUMatsue, Hideaki; Kasugai, Yoshimi; Harada, Masahide; Maekawa, Fujio; Kubo, Kenya*; Saito, Tsutomu*
no journal, ,
no abstracts in English
Hatsukawa, Yuichi; Hashimoto, Masashi; Nagai, Yasuki*; Kin, Tadahiro; Segawa, Mariko; Harada, Hideo; Konno, Chikara; Ochiai, Kentaro; Takakura, Kosuke; Iwamoto, Nobuyuki; et al.
no journal, ,
 Tc is the most important radioisotope used as nuclear medicine which is mostly produced by using only five nuclear reactors. Recently, two of the present authors proposed a new route to produce
Tc is the most important radioisotope used as nuclear medicine which is mostly produced by using only five nuclear reactors. Recently, two of the present authors proposed a new route to produce  Mo by the
Mo by the 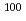 Mo(n,2n)
Mo(n,2n) Mo reaction, which has the following characteristic features. (1) First, the reaction cross section is large. Second, the cross sections of the (n,
Mo reaction, which has the following characteristic features. (1) First, the reaction cross section is large. Second, the cross sections of the (n, ), (n,np), and (n,p) reactions are less than a few mb at En = 14 MeV. Third, a large amount of
), (n,np), and (n,p) reactions are less than a few mb at En = 14 MeV. Third, a large amount of 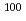 Mo target materials can be used, compared to that for proton beam irradiation on
Mo target materials can be used, compared to that for proton beam irradiation on 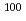 Mo. In the present work we have measured all
Mo. In the present work we have measured all  -rays emitted from activities produced by bombarding a natural Mo target with neutrons from the D(
-rays emitted from activities produced by bombarding a natural Mo target with neutrons from the D( H,n)
H,n) He reaction at FNS at Japan Atomic Energy Agency (JAEA) to study characteristic features mentioned above more in detail. The experimental results at FNS will be discussed in the conference.
He reaction at FNS at Japan Atomic Energy Agency (JAEA) to study characteristic features mentioned above more in detail. The experimental results at FNS will be discussed in the conference.
Sato, Tetsuya; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro
no journal, ,
To investigate chemical properties of the transactinide element dubnium (Db, Z=105), we have developed an on-line isothermal gas chromatographic apparatus. The on-line experiments with the group-5 elements using the short lived  Nb and
Nb and 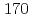 Ta as the homologues of Db were conducted. We measured the relative chemical yields of volatile compounds of
Ta as the homologues of Db were conducted. We measured the relative chemical yields of volatile compounds of 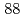 Nb and
Nb and 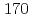 Ta as a function of the isothermal temperature in chlorinating condition with mixture of air and the SOCl
Ta as a function of the isothermal temperature in chlorinating condition with mixture of air and the SOCl vapor. It was found that the volatility of compounds of Ta is lower than that of Nb.
vapor. It was found that the volatility of compounds of Ta is lower than that of Nb.